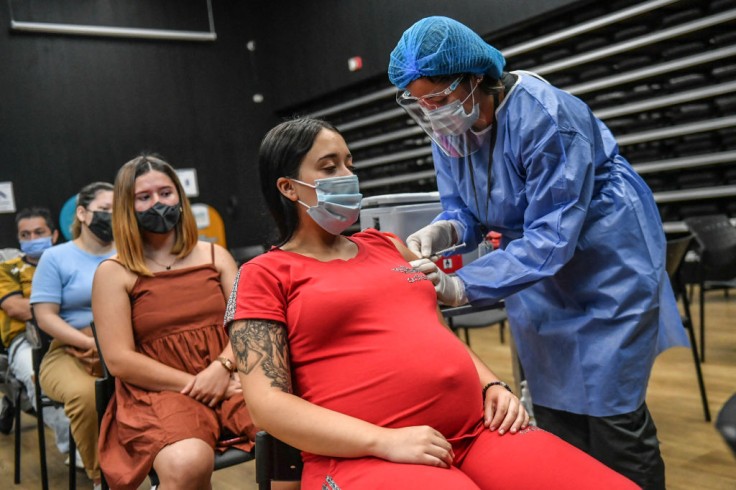
America's leading OB-GYN groups said that COVID-19 vaccination for pregnant women is imperative. The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) released a joint statement to recommend the jab as cases of the more contagious Delta variant continue to surge.
In a statement, ACOG president Dr. J. Martin Tucker said that pregnant people "should feel confident" about choosing to get vaccinated; thus, a "strong recommendation" from obstetricians and gynecologists will help in their decisions.
Before issuing an official recommendation, the groups also said that a COVID-19 vaccination should not be withheld from pregnant individuals. The latest statement has boosted studies showing that vaccines are safe and effective.
Read Also: Emmy Rossum Says New Daughter Has COVID-19 Antibodies, Urges Vaccination Even When Pregnant
ACOG and SMFM also recommended that healthcare providers continue to encourage and share information with pregnant women who may still be undecided about getting the jab, including stressing other safety protocols like mask-wearing and social distancing. The organizations also shared online information on their official sites to help members start a conversation with their patients on vaccine safety and efficacy.
COVID-19 Vaccination for Pregnant Women Endorsed
ACOG and SMFM did not endorse a particular vaccine for pregnant women. The Centers for Disease Control and Prevention (CDC) stated on its official site that studies on vaccines made in the U.S., such as Moderna, Pfizer-BioNTech, or J&J/Janssen, resulted in no safety concerns for pregnant test subjects and their babies.
The health agency also reiterated that an unvaccinated pregnant person had increased risks for severe complications brought on by the virus as the Delta variant, which has a higher transmission rate and impacts the healthcare system in the U.S.
CDC also said that COVID-19 could raise the risks of adverse outcomes during pregnancy, such as preterm births and other complications.
The U.S. Food and Drug Administration (FDA), on the other hand, said that pregnant women should discuss their options with their doctors when the agency authorized mRNA vaccines for emergency use in early 2021. However, a study published in April in the New England Journal of Medicine cited that over 35,000 pregnant women who received either Pfizer-BioNTech and Moderna vaccines did not have negative effects on the mom and their babies.
The study showed that babies inside the womb of vaccinated mothers received substantial antibodies to protect them against COVID-19. The experts said those mRNA vaccines triggered a more robust immune response as antibodies were also detected in the mother's breastmilk.
Issue with J & J Vaccine
Meanwhile, as data shows the efficacy and safety of mRNA vaccines on pregnant moms, experts are still waiting for more data for the Johnson & Johnson vaccine (J&J/Janssen). According to reports, doctors are divided on giving this vaccine because it could cause blood clots in rare instances.
However, Linda Eckert, an obstetrics and gynecology professor at the University of Washington School of Medicine, said that the reported cases linked to individuals vaccinated with J&J/Janssen occur in just seven clots per a million doses. Some pregnant patients also prefer this vaccine because it takes only a single shot for a person to be fully vaccinated, unlike mRNA vaccines that require two shots.