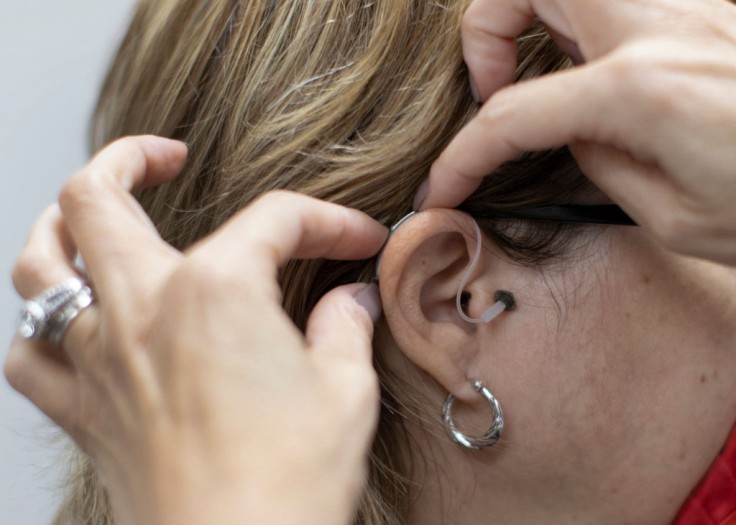
Help is on the way for Americans with hearing problems as the U.S. Food and Drug Administration (FDA) finally announced on Tuesday, August 16, a long-awaited rule change that would make hearing aids cheaper and possibly even better.
With the change, people with mild to moderate hearing loss can buy their hearing aids directly online or from a store instead of visiting a hearing health professional, getting a prescription, and then having a custom fitting.
According to FDA Commissioner Dr. Robert Califf, this move will make hearing aids much more widely available for Americans across the United States. If hearing loss was officially considered a disability by the federal government, it would be the largest disability class in the U.S., according to the U.S. Equal Employment Opportunity Commission.
Many Americans have hearing problems
About 1 in 8 people in the country ages 12 and older has hearing loss in both ears. That rate increases significantly with age. Nearly a quarter of people ages 65 to 74 have hearing loss, which goes up to 50 percent around the age of 75.
That being said, only about 16 percent of the tens of millions of Americans with hearing loss use a hearing aid. The new hearing devices won't be free, though, but the federal agency estimates that the new rule could mean savings of about $2,800 for a pair of hearing aids, according to CNN.
Califf said people could see over-the-counter hearing aids available on the market as early as October. He added that today's action by the FDA will not only help adults who have perceived mild to moderate hearing loss gain access to more affordable and innovative production options, but they expect that it will unleash the power of the U.S. industry to improve the technology in a way that it will impact the enormous burden of disability from hearing loss that is affecting the world.
Hearing aids now available over the counter
U.S. Congress passed legislation to create a category of over-the-counter hearing aids with wide bipartisan support, with President Trump signing it into law in 2017. No action was taken, though, with Senator Elizabeth Warren, a sponsor of the bill, giving the FDA until August 2020 to issue the regulations. Warren and the bill's co-sponsor Senator Chuck Grassley sent several letters urging the agency to take action.
President Biden then signed an executive order in 2021 that was created partly to speed things along. According to the FDA, the COVID pandemic delayed the rule's implementation. The agency revealed on Tuesday that it also had a lot of public comments to study before implementing the rule change.
Some hearing health associations submitted their concerns about the proposed changes during the public health comment period. They suggested that devices would still require the help of a professional.