The U.S. Food and Drug Administration (FDA) has granted approval for Litfulo, a groundbreaking treatment for teenagers battling alopecia areata.
Developed by pharmaceutical giant Pfizer, Litfulo offers new hope to young individuals coping with severe hair loss due to this autoimmune disease.
This FDA approval marks a significant milestone in the field of dermatology and paves the way for enhanced treatment options for teens facing the challenges of alopecia areata.
Alopecia Areata Treatment for Teens
The approval of Litfulo represents a significant achievement for Pfizer and the medical community at large.
The medication, also known as ritlecitinib, is poised to revolutionize the treatment landscape for alopecia areata.
Pfizer, a leading pharmaceutical company with a strong track record of innovation, has invested extensive research and development efforts to bring Litfulo to market.
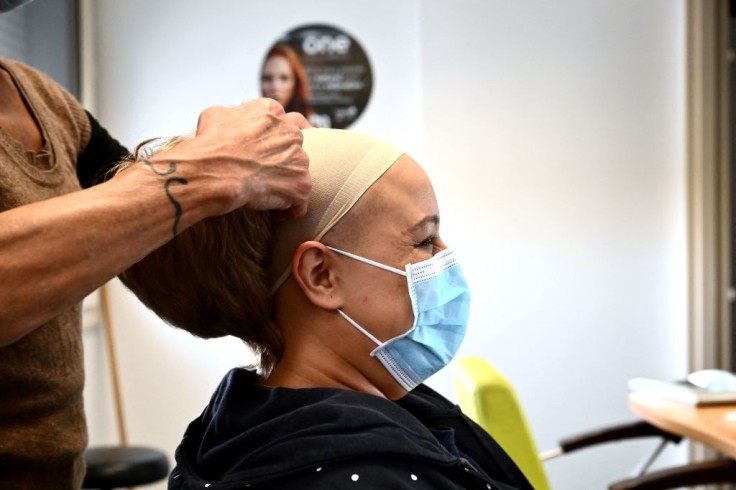
According to Good Morning America, alopecia areata is a condition in which the body's immune system mistakenly attacks the hair follicles, resulting in hair loss.
Adolescents, in particular, can face emotional and physical hardships due to the sudden and drastic change in their appearance.
However, Litfulo, a once-daily pill specifically designed for individuals aged 12 and above, aims to address these challenges and restore confidence in affected teenagers.
Clinical trials conducted at esteemed institutions, including Yale University in New Haven, Connecticut, demonstrated Litfulo's remarkable effectiveness in regrowing hair in individuals with severe alopecia areata.
Dr. Brett King, associate professor of dermatology at Yale School of Medicine, played a pivotal role as the principal investigator during these trials.
During the trials, participants with 50% to 100% scalp hair loss were administered Litfulo for 24 weeks.
The results were astounding, with approximately 30% of participants experiencing hair regrowth within this timeframe.
Notably, some individuals achieved complete scalp hair regrowth or had less than 20% hair loss after 48 weeks of treatment.
Accessing Litfulo: Cost and Availability
According to USA Today, Pfizer has committed to making Litfulo accessible to those in need.
While the list price for a full year's supply of Litfulo stands at $49,000, the actual cost to patients will vary depending on individual healthcare plans.
Recognizing the financial burden it may pose, Pfizer has implemented copay savings for commercially insured patients and a patient assistance program to facilitate access to Litfulo.
The Pfizer Dermatology Patient Access Program aims to support eligible patients by providing them with the necessary resources to access Litfulo.
This initiative aims to bridge the gap between affordability and quality care, ensuring that teenagers with alopecia areata can avail themselves of this groundbreaking treatment without undue financial strain.
The Future of Alopecia Treatment
While the approval of Litfulo marks a significant advancement in alopecia areata treatment, it is crucial to emphasize that the medication is not a cure.
Dr. Brett King, the lead investigator in the clinical trials, explained that ongoing treatment with Litfulo is necessary to maintain hair growth.
According to Business Wire, as a JAK inhibitor, Litfulo interferes with inflammatory signals in the body that contribute to alopecia areata.
However, it is important for patients with a history of cancer, blood clots, or cardiovascular disease to consult their healthcare providers before considering Litfulo.
The medication, like others in its class, has specific warnings and considerations that must be thoroughly evaluated on an individual basis to ensure patient safety.
With the FDA's approval of Litfulo, a new chapter in the treatment of alopecia areata begins.
As Litfulo becomes available in the coming weeks, teenagers and their families eagerly anticipate the opportunity to regain what alopecia took away-a sense of normalcy and renewed confidence.